Analysis of Inflammation Marker---Interleukin-6 (IL-6)
Views:790 Add time:2018-10-26 12:38:56
Interleukin-6 (IL-6) is an endogenous chemical which is active in inflammation and in B cell maturation. Besides being an immune protein, it is also a pyrogen, and is responsible for fever in autoimmune, infectious or non-infectious disease. It is produced in the body, wherever there is inflammation, either acute or chronic. It interacts with interleukin-6 receptor alpha to induce transcription of inflammatory gene products.
IL-6 is one of the key proinflammatory cytokines in triggering this response and is almost entirely responsible for inducing fever and the acute phase response in the liver. In life-threatening infections, the IL-6 level is elevated and correlates with the severity of sepsis and death rate. IL-6 levels are also raised in almost all diseases that involve chronic inflammation such as diabetes and rheumatoid arthritis.
Interleukin-6 (IL-6) has been emphasized by reports of elevated circulating as well as intracardiac IL-6 levels in patients with congestive heart failure (CHF). IL-6 may contribute to the progression of myocardial damage and dysfunction in chronic heart failure syndrome resulting from different causes. As the cause of CHF in cardiomyopathy, myocarditis, allograft rejection, and left ventricular assist device (LVADs) conditions, circulating IL-6 levels are associated with the severity of left ventricular dysfunction, and are also strong predictors of subsequent clinical outcomes.
Anti-HumanIL-6 Monoclonal Antibodies
Four anti-human IL-6 monoclonal antibodies wererecentlydeveloped by CUSAg, which make possible the development of highly sensitive and rapid sandwich immunoassays.Our in-house assays have a linear detection range from 0~1500 pg/mL. All recommended MAb combinations were evaluated in medium-scale clinical trials with blood samples fromnormal and patients withchronic infections, autoimmune disorders, certain cancers, and Alzheimer’s disease.
|
Properties |
Specification |
|
Target species |
Human |
|
Host animal |
Mice Balb/c |
|
Cell line used for fusion |
Sp2/0 |
|
Immunogen |
Human IL-6 |
|
Purification method |
Protein G affinity chromatography |
|
Presentation |
MAb solution in PBS ( pH 7.4) |
|
Application |
CLIA and others |
|
Catalog Number |
CSB-DA436EmN① CSB-DA436EmN② CSB-DA436EmN③ CSB-DA436EmN④ |
Calibration Curve
All MAbs were tested in pairs as capture and detection antibodies to select the best two-site MAb combinations for the development of a quantitative sandwich immunoassay. Calibration curves for several best two-site combinations, which utilized different anti-IL-6 MAbs, are shown in Fig.1. Detection antibodies were labeled withhorseradish peroxidase (HRP). The best selected MAb combinations for the development of quantitative human IL-6 immunoassays are (capture-detection respectively):
MAb combination A: CSB-DA436EmN①- CSB-DA436EmN④;
MAb combination B: CSB- DA436EmN②- CSB-DA436EmN④;
MAb combination C: CSB- DA436EmN③-CSB-DA436EmN④;
MAb combination D: CSB- DA436EmN④- CSB-DA436EmN②.
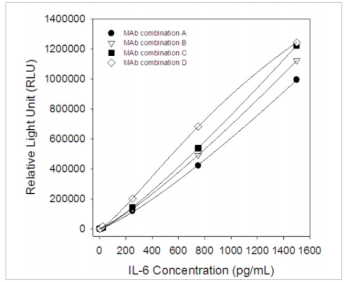
Fig.1 Six-pointcalibration curve forIL-6 sandwichchemiluminescence immunoassay (Calibrator concentrations: 0, 2.5, 25, 250, 750, 1500 pg/mL)
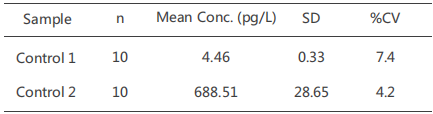
Precision
One high control (700 pg/mL) and one low control (5 pg/mL) were evaluated byour CUSAg CLIA IL-6 assay using the MAb combinationBin 10 consecutive runs, the results are summarised in the table below:
Recovery
Known concentrations of IL-6 were added to five aliquots of human serum. The concentration of IL-6 was determined by CUSAg CLIA platform and the resulting percent recovery was calculated. The recovery percentage mean valueof theIL-6immunoassay usingMAb combinationB was 87.0%.
Clinical Comparison
23 samples from apparentlyhealthydonors and patients with chronic infections were detected with CUSAgCLIAIL-6assays using MAb combination B. As shown in Fig.2, Thecorrelation coefficient between ourhome-grown assay and comparison assay is over 0.97, these results reveal these monoclonal antibodies can be applied todouble-MAb-sandwich-immunoassays.
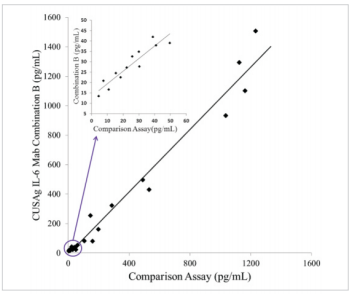
Fig.2 Comparison ofCUSAgIL-6 CLIA andcommercial diagnosticassays
Reference
1.Nishimoto N (May 2006). Interleukin-6 in rheumatoid arthritis. Current Opinion in Rheumatology. 18 (3): 277–81.
2.Holven KB, Aukrust P, Retterstol K, Hagve TA, Mørkrid L, Ose L, Nenseter MS. Increased levels of C-reactive protein and interleukin-6 in hyperhomocysteinemic subjects. Scand J Clin Lab Invest. 2006;66(1):45-54.
3.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men.Circulation. 2000 Apr 18;101(15):1767-72.
4.Tadamitsu Kishimoto.IL-6: from its discovery to clinical applications. Int. Immunol. (2010)

